Monoclonal Antibodies Developed
for SARS-CoV-2 Detection
The novel coronavirus has infected almost a million people and killed tens of thousands on nearly every continent. As there is no specific medicine to prevent or treat COVID-19, the early identification and quarantine of infected people has become the most feasible way to contain the pandemic spread. Therefore, the rapid and straightforward point-of-care tests are of vital importance. Bioss is a research-driven company dedicated to the production of high-quality antibodies. As part of the collective effort in fighting against COVID-19, Bioss has developed multiple monoclonal antibodies specific to SARS-CoV-2 proteins, for the following 3 applications.
application 1: Research Antibodies
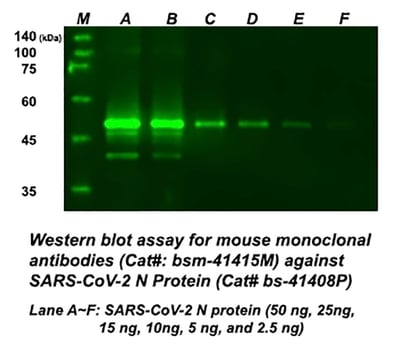
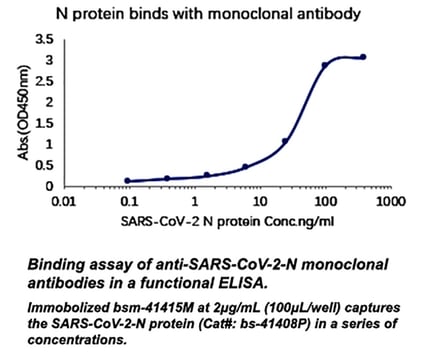
Monoclonal research antibodies validation in WB and ELISA
See full COVID19 related product list here
application 2: ELISA Kit for SARS-CoV-2 Research
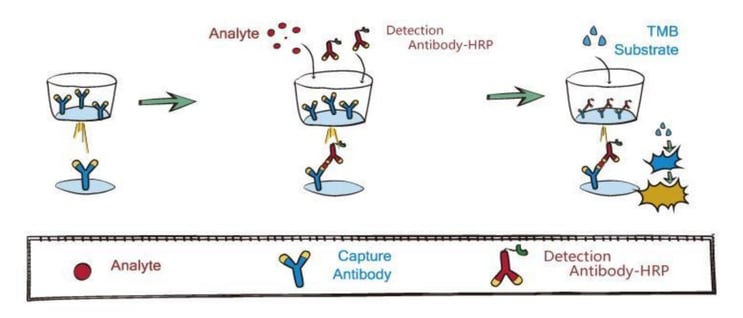
We employ the quantitative sandwich ELISA to develop this kit. Briefly, a monoclonal antibody specific for the nucleocapsid protein (N-protein) of SARS-CoV-2 was pre-coated onto a microplate. Samples of the standard and assays are added into wells, and then the HRP-conjugated detection antibody that recognizes the different epitope of N-proteins is added into wells to form an antibody-antigen-antibody "sandwich complex". Following the incubation and washes, the substrate of HRP will be added, and the resulting products are yellowish. The darkness of the color shows positive correlation with the amount of N-protein. A standard curve is plotted to determine the concentration of N-proteins in assay samples.
application 3: POCT (point-of-care test) Kit for SARS-CoV-2 Detection
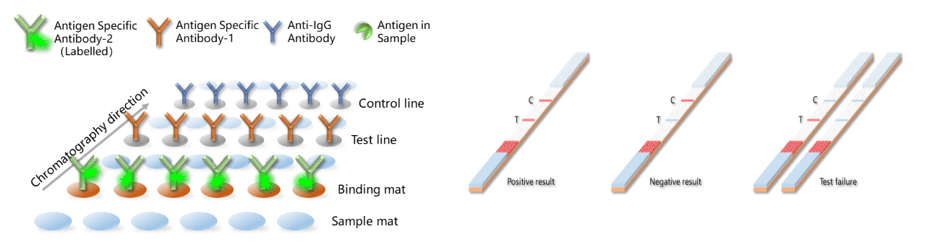
The ultimate goal of Bioss is to improve the health and quality of life of humans and animals. Facing the worldwide pandemic, we initiated the development of POC tests immediately. With our expertise, multiple monoclonal antibodies have been successfully developed. In collaboration with an IVD (in vitro diagnostic) company, these antibodies have been made into IVD kits that have obtained the CE marking. Meanwhile, there are several ongoing collaborations between Bioss and IVD companies in Europe and the United States.
Validated antibody pairs and antibody-antigen samples are available for diagnostic companies for testing, please email support@biossusa.com to request.